Antidepressants & ARVs
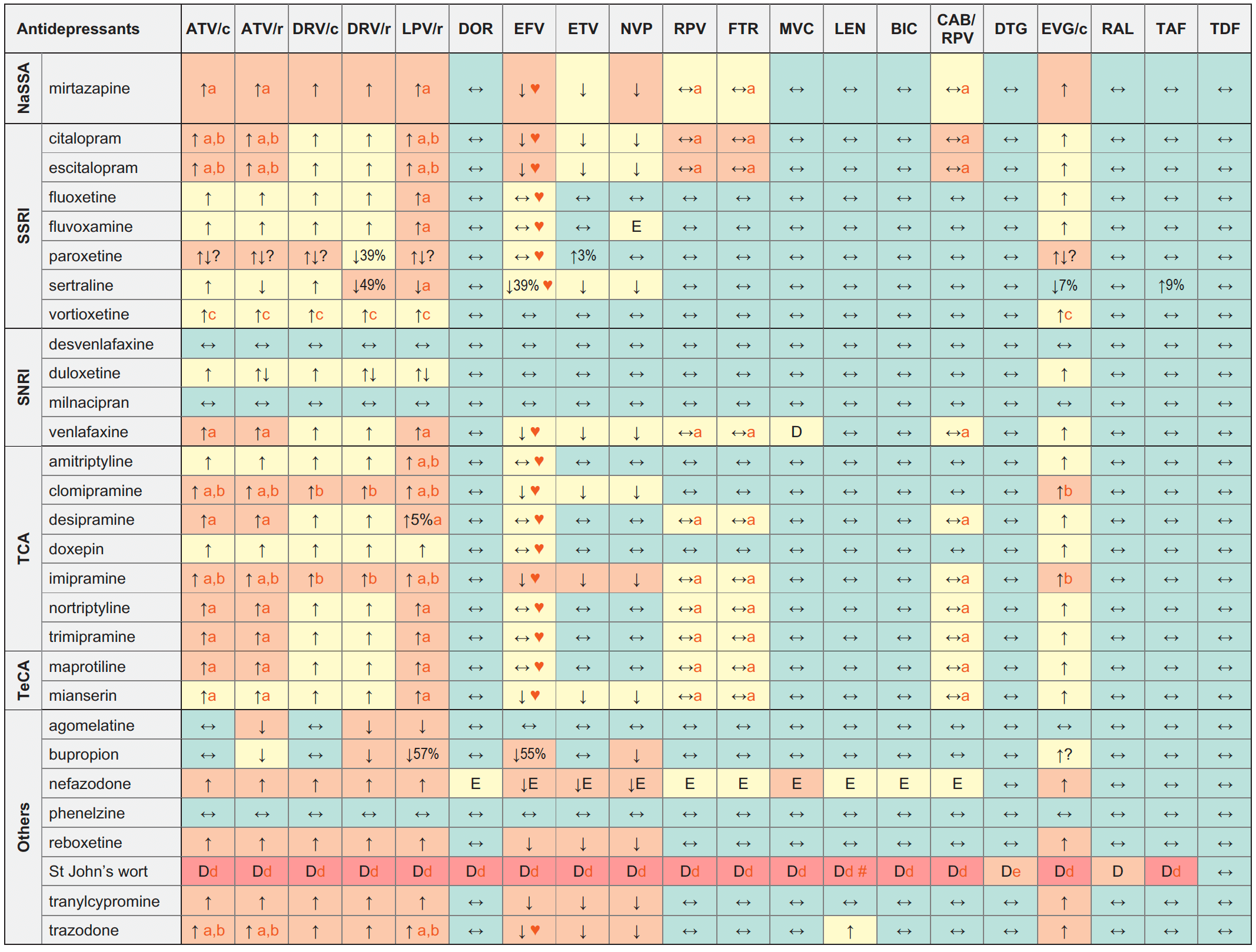
Legend
↑ Potential elevated exposure of the antidepressant
↓ Potential decreased exposure of the antidepressant
↔ No significant effect
D Potential decreased exposure of ARV drug
E Potential elevated exposure of ARV drug
Numbers refer to increased or decreased AUC as observed in drug-drug interaction studies
ATV/c:
ATV co-formulated with COBI (300/150 mg qd)
DRV/c:
DRV co-formulated with COBI (800/150 mg qd)
CAB/RPV:
CAB and RPV im long acting injections
NaSSA (noradrenergic specific serotonergic antidepressant)
SSRI (selective serotonin reuptake inhibitors)
SNRI (serotonin and norepinephrine reuptake inhibitors)
TCA (tricyclic antidepressants)
TeCA (tetracyclic antidepressants)
Interactions with ABC, FTC, 3TC, ZDV
ABC, FTC, 3TC, ZDV:
No clinically relevant interactions expected
Interactions with cabotegravir (oral)
None
Interactions with ibalizumab
None
Comments
- Caution as both drugs can induce QT interval prolongation.
- ECG monitoring is recommended.
- Based on the patient clinical response, a lower dose of vortioxetine may be needed in poor CYP2D6 metabolizers in the presence of a strong CYP3A4 inhibitor.
- A study suggests a low risk of a clinically relevant pharmacokinetic interaction with low-hyperforin formulations (< 1 mg/day) of St John’s Wort (hyperforin is the constituent responsible for induction of CYPs and P-gp). Co-administration may be considered with St John’s Wort formulations that clearly state the hyperforin content and which have a total daily hyperforin dose of 1 mg or less.
- The European SmPC recommends DTG 50 mg bid in persons without INSTI resistance. The US Prescribing Information recommends that co-administration should be avoided as there are insufficient data to make dosing recommendations.
♥ EFV prolonged the QT interval above the regulatory threshold of concern in homozygous carriers of the CYP2B6*6/*6 allele (516T variant). Coadministration with a drug with a known risk of TdP is contraindicated in the EFV European label.
# At least a 2-week (moderate inducers) or 4-week (strong inducers) cessation period is recommended prior to initiation of LEN due to the persisting inducing effect after discontinuation of an inducer.
Further Information
For additional drug-drug interactions and for more detailed pharmacokinetic interaction data and dosage adjustments, please refer to: http://www.hiv-druginteractions.org (University of Liverpool)
