Metabolic Dysfunction-Associated Steatotic Liver-Disease (MASLD)
Non-alcoholic fatty liver disease (NAFLD) is characterized by fatty infiltration of the liver (hepatic steatosis involving > 5% of hepatocytes) either on liver histology or imaging. Experts proposed redefining NAFLD as metabolic dysfunction-associated steatotic liver disease (MASLD) to provide a positive rather than exclusionary diagnosis. However, the role of contemporary ART on MASLD (in particular regarding an association with weight gain) remains unknown.
To be diagnosed with MASLD, a person must not have a history of heavy alcohol use, while coexistence of HCV or HBV is not an exclusionary criterion for MASLD.
It is often associated with components of the metabolic syndrome: overweight, type 2 diabetes, dyslipidemia and hypertension. However, MASLD can occur in lean patients (BMI<25). MASLD may affect 25% of lean people with HIV.
There are two types of MASLD:
1. Metabolic dysfunction-associated steatotic liver (MASL), with steatotic liver but no inflammation
2. Metabolic dysfunction-associated steatohepatitis (MASH), with steatotic infiltration along with liver inflammation (hepatocyte injury with or without fibrosis)
The prevalence of MASLD is higher in persons with HIV (30 - 40%) than in the general population. Nearly half of the persons with HIV who undergo evaluation for unexplained liver test abnormalities are found to have MASLD.
Metabolic dysfunction-associated steatohepatitis (MASH)
- Early MASH: no or mild (F0-F1) fibrosis
- Fibrotic MASH: significant (≥ F2) or advanced (≥ F3, bridging) fibrosis
- MASH-cirrhosis (F4)
- HCC (can occur in the absence of cirrhosis and histological evidence of MASH)
Diagnosis
- Ultrasound is the preferred first-line diagnostic procedure for imaging of MASLD
- Whenever imaging tools are not available or feasible, serum biomarkers and scores are an acceptable alternative for the diagnosis
- Where available and in experienced centres, transient elastography with controlled attenuation parameter could be used to diagnose HIV-associated MASLD, although no optimal cut-off has been established yet. Few studies have validated CAP cut-off in HIV-associated MASLD using different values (248 dB/m or 285 dB/m)
- A quantitative estimation of liver fat can only be obtained by MR spectroscopy as well as MRI-PDFF. This technique is of value in clinical trials and experimental studies but is expensive and not recommended in the clinical setting
- MASH has to be diagnosed by a liver biopsy showing steatosis, hepatocyte ballooning and lobular inflammation
Consideration of ARV drugs
-
Consider use of metabolic neutral ART regimens in individuals at risk of or with MASLD (e.g. risk of weight gain induced by INSTI or TAF)
Treatment of MASLD
- Lifestyle modification and weight reduction is the cornerstone of treatment
- Dietary restriction PLUS progressive increase in aerobic exercise/resistance training: Caloric restriction (500-1,000 kcal/day) targeting 7-10% weight loss in persons with central obesity and/or overweight; 150-200 min/ week of moderate intensity aerobic physical activities in 3-5 sessions
- A Mediterranean diet should be advised to improve steatosis and insulin sensitivity
- Pharmacotherapy should be reserved for individuals with MASH, particularly for those with significant fibrosis ≥ F2 and individuals with less severe disease, but at high risk of faster disease progression (i.e. with diabetes, metabolic syndrome, persistently increased ALT, high necroinflammation)
- Management of MASH should be discussed with hepatologists. Options with proven efficacy include pioglitazone, vitamin E and bariatric surgery. In the specific setting of HIV-associated MASLD, tesamorelin and vitamin E have demonstrated efficacy, however larger studies are needed. Researchers advocate for inclusion of persons with HIV in ongoing global trials of new antifibrotic molecules for MASH
- Statins may be safely used but have demonstrated no impact on MASLD thus far. The same is true for n-3 polyunsaturated fatty acids
Diagnostics
Diagnostic Flow-chart to Assess and Monitor Disease Severity in Case of Suspected MASLD and Metabolic Risk Factors
Persons with HIV at risk of MASLD(i)
(any among MASLD suggested by ultrasound, overweight, metabolic syndrome, persistent elevation of ALT, exposure to d-drugs)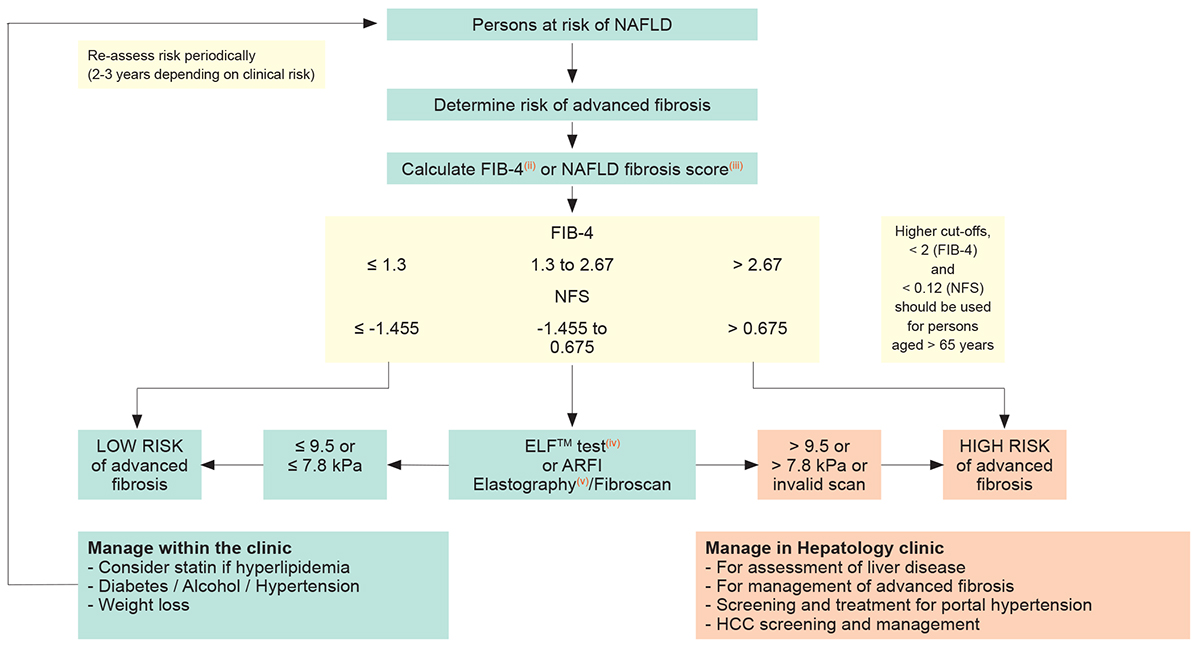
These recommendations are largely inspired by the EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease: European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity
- MASLD, metabolic dysfunction-associated steatotic liver disease
- FIB-4 = Age ([years] x AST [U/L]) / platelet [109/L] x ALT [U/L])
- NFS, Non-alcoholic fatty liver disease Fibrosis Score = -1.675 + 0.037 x age (years) + 0.094 x BMI (kg/m2) + 1.13 x impaired fasting glucose/diabetes mellitus(iv) (yes = 1/ no = 0) + 0.99 x AST/ALT ratio-0.013 x platelet (x109)-0.66 x albumin(g/dL)
- ELFTM test, Enhanced Liver Fibrosis Test is a blood test that provides an estimate of liver fibrosis severity by measuring Hyaluronic Acid (HA), Amino-terminal propeptide of type III procollagen (PIIINP), Tissue inhibitor of metalloproteinase 1 (TIMP-1)
- ARFI elastography, Acoustic Radiation Force Impulse
