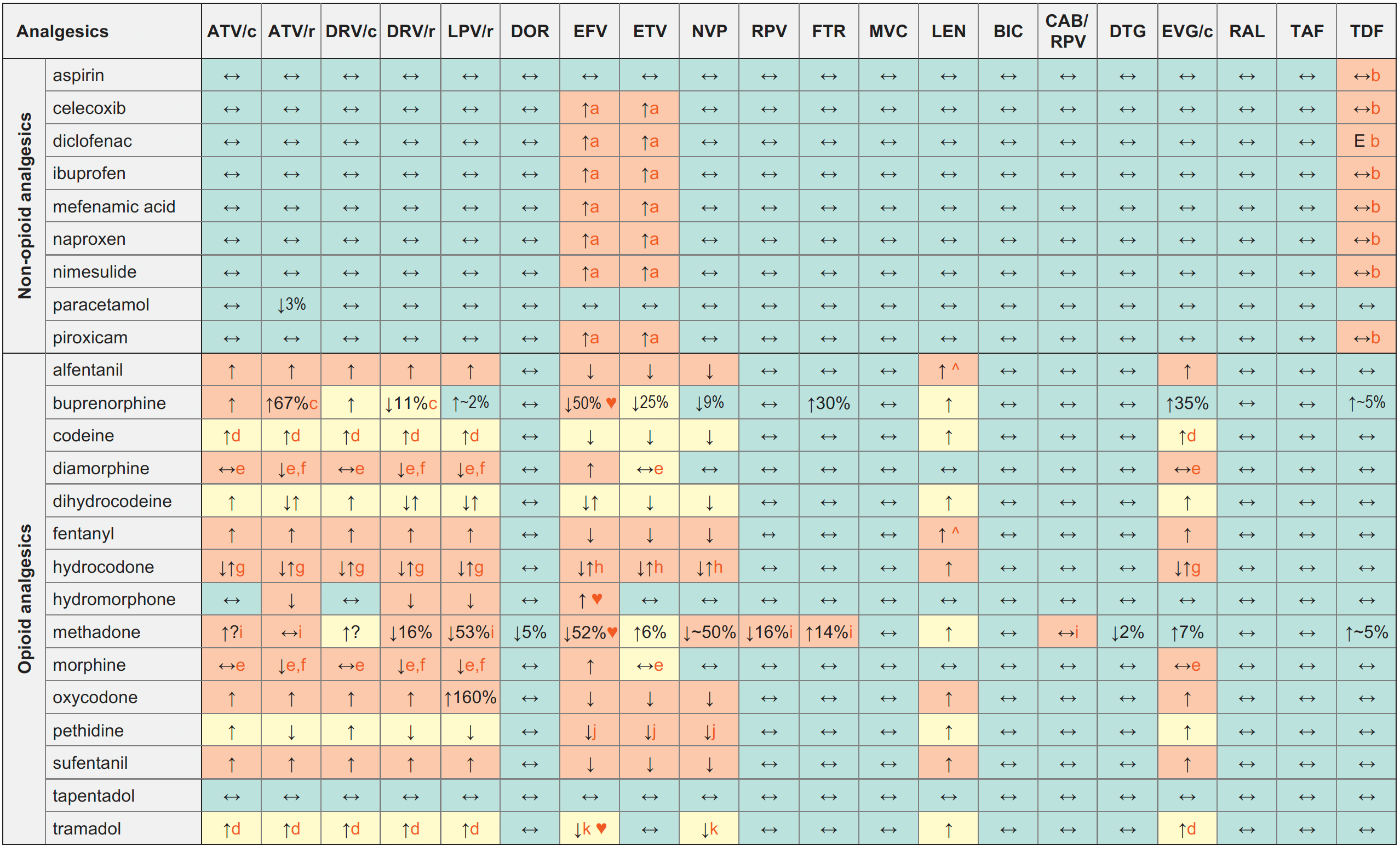
Analgesics & ARVs

Legend
↑ Potential elevated exposure of the analgesic
↓ Potential decreased exposure of the analgesic
↔ No significant effect
D Potential decreased exposure of ARV drug
E Potential elevated exposure of ARV drug
Numbers refer to increased or decreased AUC as observed in drug-drug interaction studies
ATV/c:
ATV co-formulated with COBI (300/150 mg qd)
DRV/c:
DRV co-formulated with COBI (800/150 mg qd)
CAB/RPV:
CAB and RPV im long acting injections
Interactions with ABC, FTC, 3TC, ZDV
ABC:
Decreased methadone exposure
FTC, 3TC:
No clinically relevant interactions expected
ZDV:
- Potential additive haematological toxicity with aspirin, celecoxib, diclofenac, ibuprofen, mefenamic acid, naproxen, nimesulide, piroxicam;
- Moderately increased ZDV exposure with methadone; monitor for toxicity
Interactions with cabotegravir (oral)
None
Interactions with ibalizumab
None
Comments
- Clinical significance unknown. Use the lowest recommended dose particularly in individuals with risk factors for CVD, those individuals at risk of developing gastrointestinal complications, persons with hepatic or renal impairment, and in elderly persons.
- Potential risk of nephrotoxicity which is increased if NSAID is used for a long duration, if the person has a pre-existing renal dysfunction, a low body weight or receives other drugs that may increase TDF exposure. Concurrent use of NSAIDs with TDF warrants monitoring of renal function.
- Concentrations of norbuprenorphine increased.
- Potential decrease of the analgesic effect due to the reduced conversion to the active metabolite.
- Inhibition of P-gp by RTV, COBI or ETV could potentiate the effect of opiate in the CNS.
- Concentrations of parent drug decreased but concentrations of active metabolite increased.
- Concentrations of hydrocodone increased, but concentrations of active metabolites (norhydrocodone and hydromorphone) decreased. The clinical significance of this is unclear.
- Concentrations of hydrocodone decreased, but concentrations of norhydrocodone increased. The clinical significance of this is unclear.
- Both drugs can potentially prolong the QT interval, ECG monitoring recommended.
- Concentrations of parent drug decreased and concentrations of neurotoxic metabolite increased.
- Concentrations of parent drug decreased but no change in concentrations of more active metabolite.
♥ EFV prolonged the QT interval above the regulatory threshold of concern in homozygous carriers of the CYP2B6*6/*6 allele (516T variant). Coadministration with a drug with a known risk of TdP is contraindicated in the EFV European label.
^ LEN causes moderate inhibition of CYP3A4 and, when discontinued, remains in the circulation for prolonged periods. Residual concentrations of LEN may affect the exposure of sensitive CYP3A4 substrates and/or narrow therapeutic index drugs that are initiated within 9 months after the last subcutaneous dose of LEN.
Further Information
For additional drug-drug interactions and for more detailed pharmacokinetic interaction data and dosage adjustments, please refer to: http://www.hiv-druginteractions.org (University of Liverpool)
