ARVs: Swallowing Difficulties
Administration of ARVs in Persons with Swallowing Difficulties
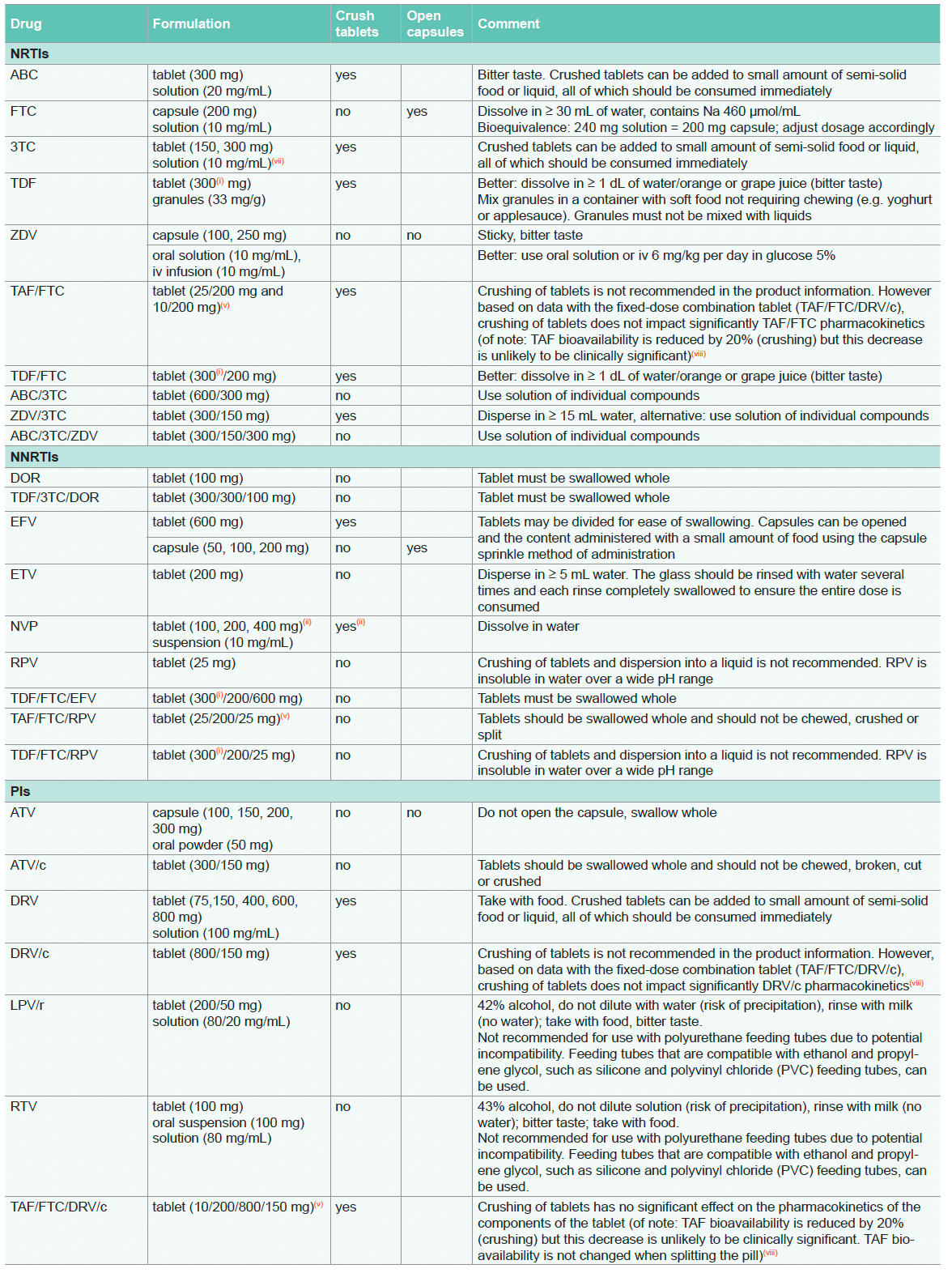
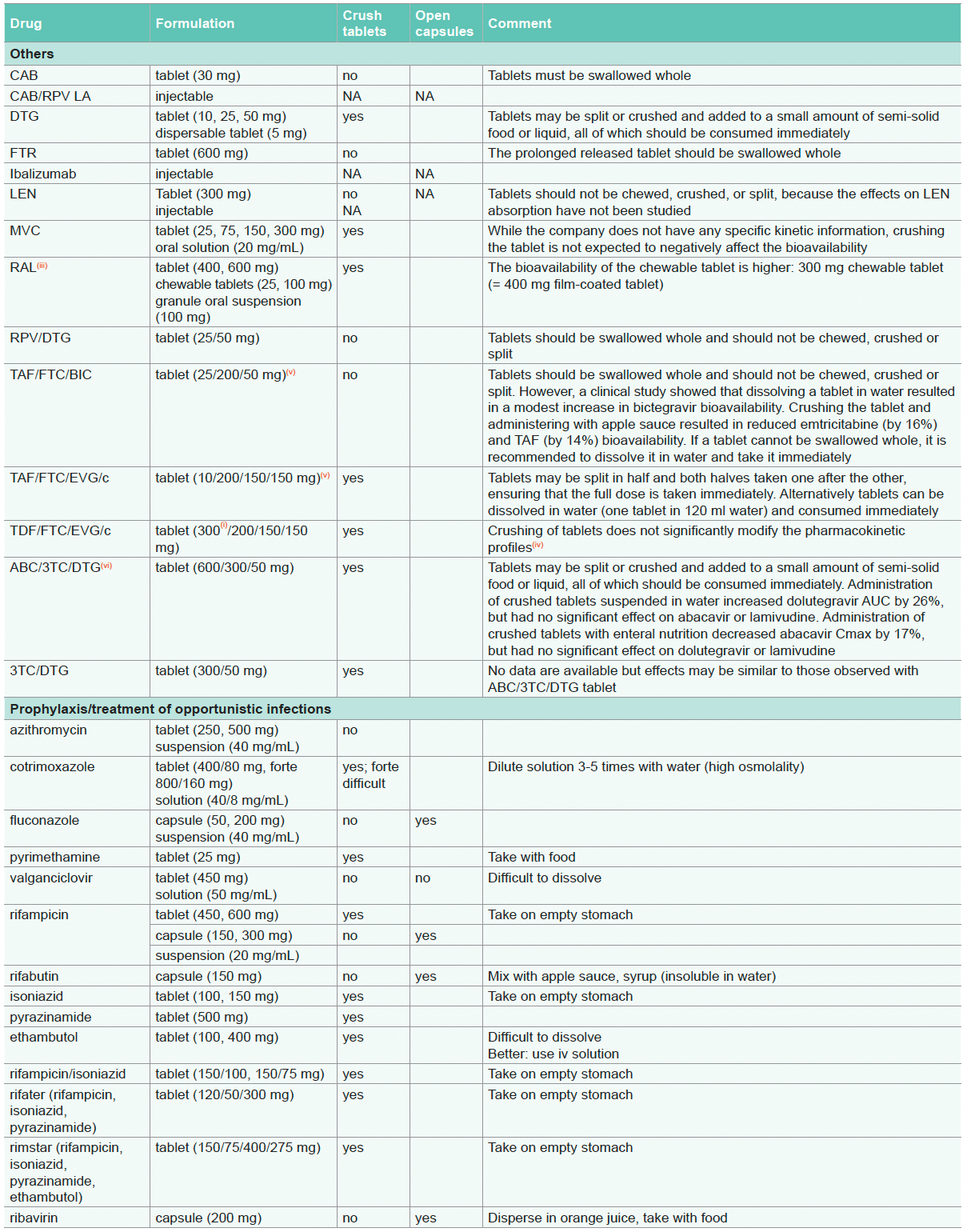
For recommendations on prophylaxis/treatment of opportunistic infections, see Opportunistic Infections
- In certain countries TDF is labelled as 245 mg rather than 300 mg to reflect the amount of the prodrug (tenofovir disoproxil) rather than the fumarate salt (tenofovir disoproxil fumarate). The 245 mg dose is equivalent to 7.5 scoops of granules
- Extended release effect lost. Note: NVP 400 mg qd (immediate release) can lead to sub-therapeutic trough levels in individuals with higher body weight (≥ 90 kg) compared to NVP 200 mg bid. Therefore, NVP bid administration should be preferred in individuals with higher body weight
- Crushing tablets is not recommended in the product information, however absorption of RAL was not compromised when the drug was crushed, dissolved in 60 mL warm water and administered by gastrostomy tube. In addition, RAL drug absorption has been shown to be higher in persons taking RAL 400 mg bid by chewing the tablets as compared to swallowing the intact tablets
- Crushing tablets is not recommended in the product information however the pharmacokinetic profiles of TDF/FTC/EVG/c were not significantly modified when the fixed-dose combination tablet (Stribild) was crushed and administered with food or with drip feed compared to the administration of the whole tablet
- TAF is used at 10 mg when co-administered with drugs that inhibit P-gp. TAF is used at 25 mg when co-administered with drugs that do not inhibit P-gp
- The pharmacokinetic profiles of ABC/3TC/DTG were not modified to a clinically significant extent when the fixed-dose combination tablet (Triumeq) was crushed and administered suspended in water or in enteral nutrition (of note: crushing leads to a 26% increase in DTG exposure)
- The bioavailability of 3TC solution has been shown to be significantly reduced in a dose dependent manner by sorbitol present in other liquid formulations (e.g. ABC, NVP, cotrimoxazole)
- Crushing of tablets is not recommended in the product information, however the individual pharmacokinetic profiles of TAF/FTC/ DRV/c were not significantly modified when the fixed-dose combination tablet (Symtuza) was administered crushed or split compared to the whole tablet
