DDIs, Prescribing, Overview
Drug-drug Interactions and Other Prescribing Issues
ARVs are recognised to be amongst the therapeutic agents with the highest potential for drug-drug interactions (DDIs) as these drugs can be both a victim (affected by other drugs) and/or a perpetrator (affect other drugs) of DDIs. Given the life-long ART, DDIs are practically unavoidable in persons with HIV and comorbid conditions. Thus, the potential for DDIs should be considered systematically when selecting an ART regimen or when any new medicine is coadministered to existing ART with particular attention to adjust dosage and perform clinical monitoring when needed.
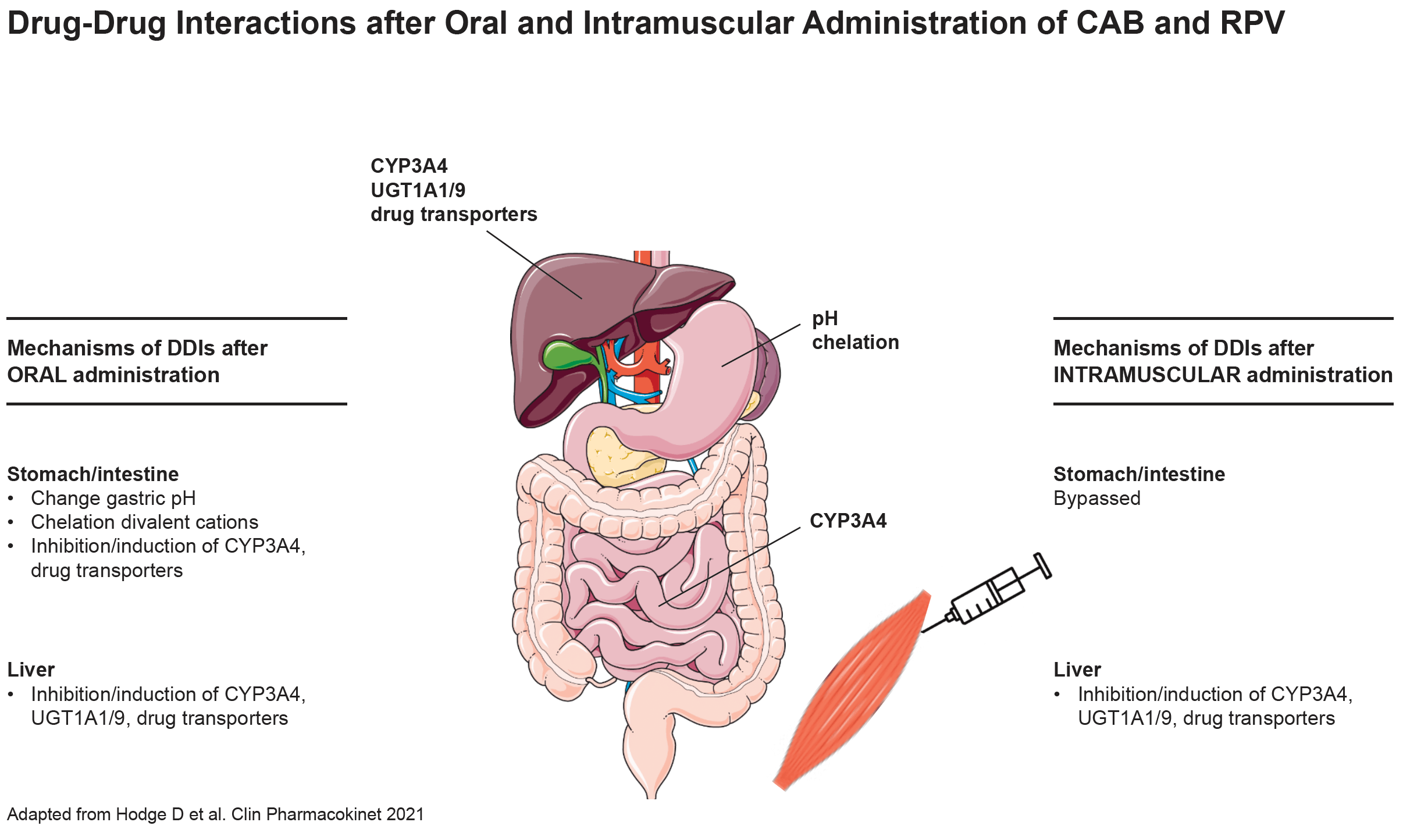
The im administration of the ARVs CAB and RPV presents the advantage of eliminating DDIs occurring at the gastrointestinal level due to changes in gastric pH (rilpivirine requires a low pH for optimal absorption); chelation (cabotegravir forms a complex with divalent cations thereby impairing its absorption) or inhibition/induction of intestinal drug metabolizing enzymes. However, escaping the first-pass metabolism does not necessarily mitigate the DDI magnitude if the drug is minimally metabolized in the gut such as cabotegravir. Conversely, the magnitude of DDIs is mitigated for rilpivirine due to its high first-pass metabolism [Bettonte S et al. Clin Infect Dis 2023]. Regardless, strong and moderate inducers are predicted to cause a significant decrease in cabotegravir and rilpivirine exposure after intramuscular administration and therefore coadministration is not recommended with inducers.
| Examples of medications interacting with the oral but not the intramuscular administration of RPV | Examples of medications interacting with the oral but not the intramuscular administration of CAB |
|
• Antacids |
• Antacids • calcium • iron • magnesium • multivitamins containing divalent cations • orlistat • strontium ranelate |
Comments
- The rich vascular supply of the muscle favors the drug release from the depot therefore the injection technique is critical to ensure that cabotegravir and rilpivirine are not deposited in the subcutaneous adipose tissue (where blood flow and release from the depot are reduced leading potentially to lower initial drug concentrations). Thus, a longer needle is recommended when administering cabotegravir/rilpivirine to individuals with a BMI ≥ 30 kg/m2. Another factor that could potentially enhance the drug release from the depot includes high physical activity as it increases the blood flow in the muscle.
- Multivariate models using data from phase 3 trials have shown that a combination of ≥ 2 baseline factors (including pre-existing rilpivirine RAMs, HIV subtype A6/A1 and/or BMI ≥ 30 kg/m2) increased the risk of virologic failure. Orkin C et al. Clin Infect Dis 2023.
- Dosing recommendations in case of missed injections: doses can be administered between 7 days before and 7 days after the dose is due. If a 2-monthly injection is missed by < 2 months, treatment can be resumed as normal; however, if it is missed by > 2 months, cabotegravir/ rilpivirine 600/900 mg dose must be administered as soon as possible, followed by a second dose after 4 weeks, before treatment may continue as normal. If an individual plans to miss a scheduled injection visit by more than 7 days, oral cabotegravir/rilpivirine 30/25 mg once daily may be used for up to 2 months to replace one missed injection visit (every 2-month dosing schedule).
The DDIs profiles between ARVs and coadministered medicines within a therapeutic class are also presented in the corresponding Co-morbidities section and Viral Hepatitis Co-infection section
Detailed information on DDIs can be found on the University of Liverpool DDIs websites: http://www.hiv-druginteractions.org and http://www.hep-druginteractions.org
Real-life experiences on the clinical management of drug-drug interactions can be found in: clinicalcasesddis.com
Age-related physiological changes and co-morbidities predispose older persons with HIV to inappropriate drug use or dosing in addition to DDIs.
Besides highlighting the most common DDIs, this section also provides guidance on how to adjust drug dosing in case of liver or renal impairment, considerations for those with swallowing difficulties and what to consider when prescribing drugs in older persons with HIV including drug classes to avoid or to deprescribe in the presence of certain conditions.