Pulmonary Antihypertensives & ARVs
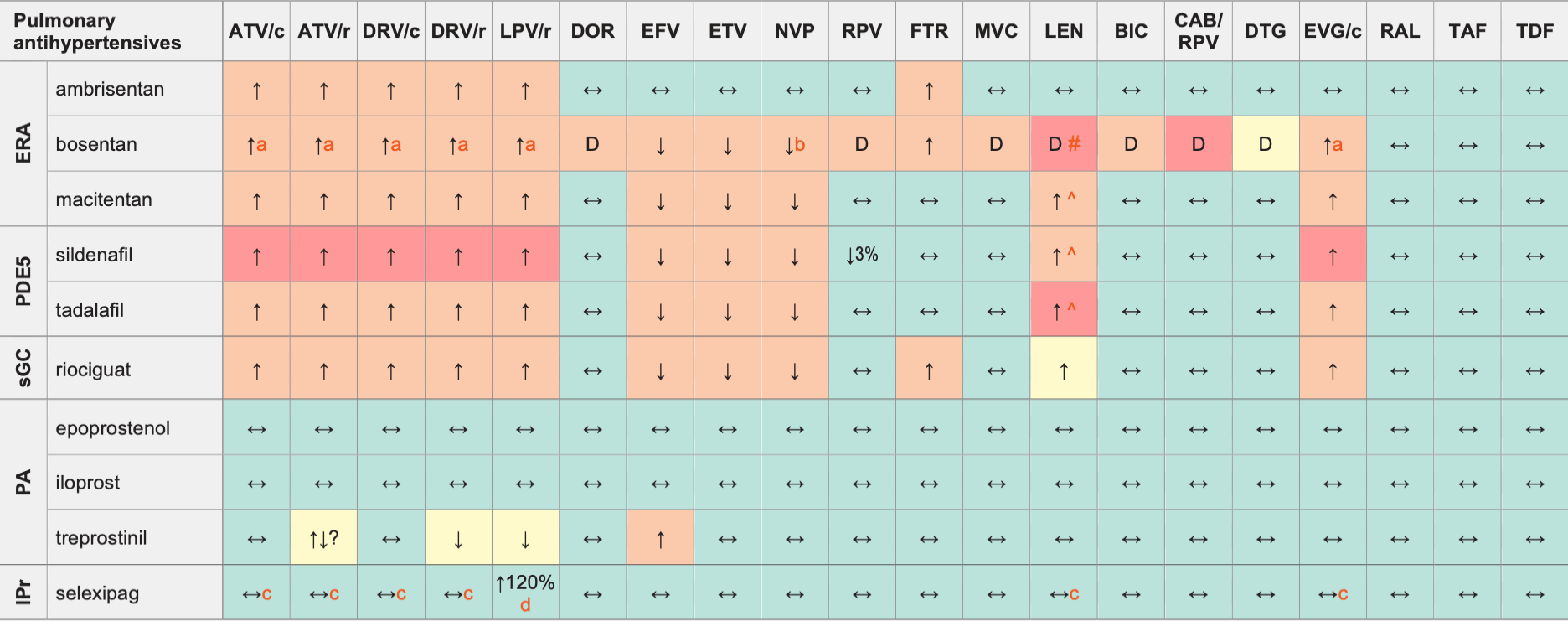
Legend
↑ Potential elevated exposure of the pulmonary antihypertensive
↓ Potential decreased exposure of the pulmonary antihypertensive
↔ No significant effect
D Potential decreased exposure of ARV drug
E Potential elevated exposure of ARV drug
Numbers refer to increased or decreased AUC as observed in drug-drug interaction studies
ATV/c:
ATV co-formulated with COBI (300/150 mg qd)
DRV/c:
DRV co-formulated with COBI (800/150 mg qd)
CAB/RPV:
CAB and RPV im long acting injections
ERA (endothelin receptor antagonists)
Ipr (IP receptor agonists)
PA (prostacyclin analogues)
PDE5 (phosphodiesterase type 5 inhibitors)
sGC (soluble guanylate cyclase stimulators)
Interactions with ABC, FTC, 3TC, ZDV
ABC, FTC, 3TC, ZDV:
No clinically relevant interactions expected
Interactions with cabotegravir (oral)
None
Interactions with ibalizumab
None
Comments
- Co-administration is not recommended in the European labels, but the US labels suggest the following dose modifications: When starting bosentan in persons already on PI/b or EVG/c use a bosentan dose of 62.5 mg qd or every other day. Discontinue bosentan at least 36 h prior to starting PI/b or EVG/c and restart after at least 10 days at 62.5 mg qd or every other day.
- Potential additive liver toxicity.
- Exposure of parent drug increased but exposure of active metabolite unchanged.
- This change is unlikely to be clinically relevant.
^ LEN causes moderate inhibition of CYP3A4 and, when discontinued, remains in the circulation for prolonged periods. Residual concentrations of LEN may affect the exposure of sensitive CYP3A4 substrates and/or narrow therapeutic index drugs that are initiated within 9 months after the last subcutaneous dose of LEN.
# At least a 2-week (moderate inducers) or 4-week (strong inducers) cessation period is recommended prior to initiation of LEN due to the persisting inducing effect after discontinuation of an inducer.
Further Information
For additional drug-drug interactions and for more detailed pharmacokinetic interaction data and dosage adjustments, please refer to: http://www.hiv-druginteractions.org (University of Liverpool)
