Viral Hepatitis Drugs & ARVs
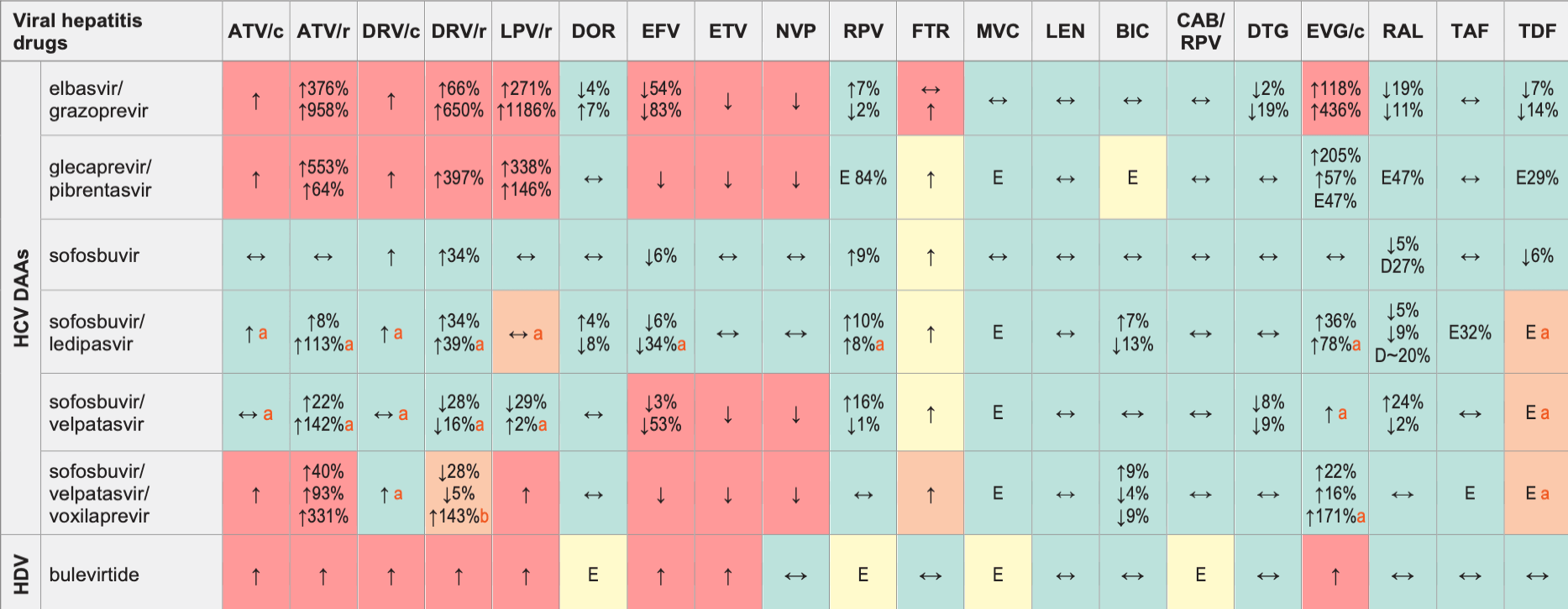
Legend
↑ Potential elevated exposure of the hepatitis therapy
↓ Potential decreased exposure of the hepatitis therapy
↔ No significant effect
D Potential decreased exposure of ARV drug
E Potential elevated exposure of ARV drug
Numbers refer to increased or decreased AUC as observed in drug-drug interaction studies.
First/second numbers refer to AUC changes for EBR/GZR or GLE/PIB or SOF/LDV or SOF/VEL.
First/second/third numbers refer to AUC changes for SOF/VEL/VOX.
ATV/c:
ATV co-formulated with COBI (300/150 mg qd)
DRV/c:
DRV co-formulated with COBI (800/150 mg qd)
CAB/RPV:
CAB and RPV im long acting injections
Interactions with ABC, FTC, 3TC, ZDV
ABC, FTC, 3TC, ZDV:
No clinically relevant interactions expected
Interactions with cabotegravir (oral)
None
Interactions with ibalizumab
None
Comments
- Monitoring of renal function recommended due to increase of tenofovir concentration if the regimen contains TDF.
- Study details are with DRV/r qd. DRV bid has not been studied and should be used with caution as voxilaprevir concentrations may increase more than with DRV qd (this would be of further significance in cirrhotic patients). Monitoring of renal function recommended due to increase of tenofovir concentrations if the regimen contains TDF.
Further Information
For additional drug-drug interactions and for more detailed pharmacokinetic interaction data and dosage adjustments, please refer to: http://www.hiv-druginteractions.org (University of Liverpool)
