Contraceptives & ARVs
Legend
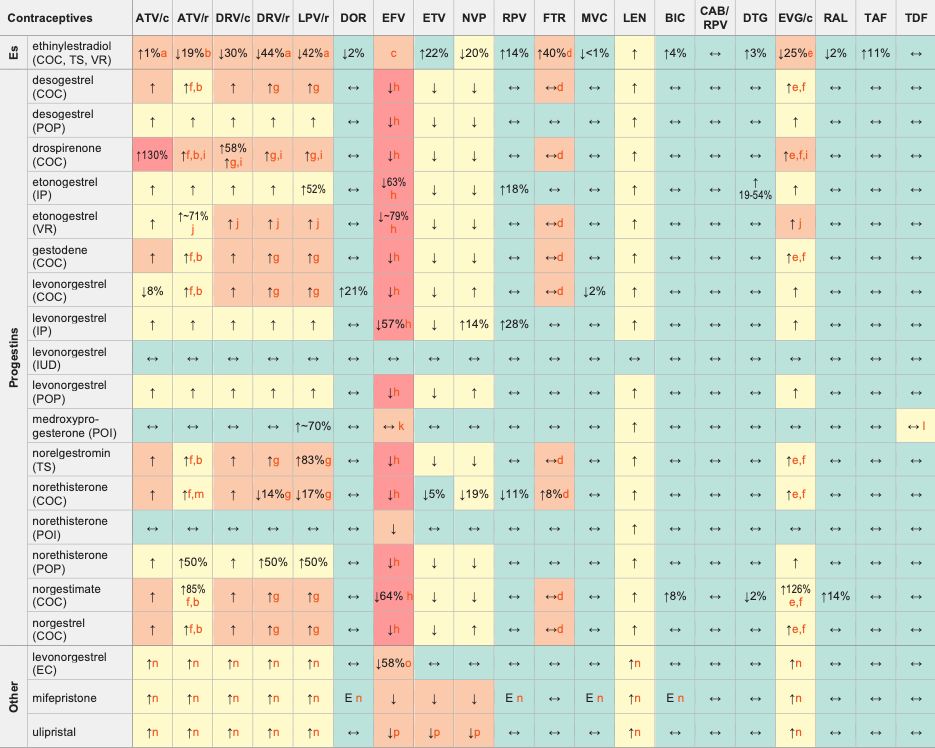
↑ Potential elevated exposure of the hormone
↓ Potential decreased exposure of the hormone
↔ No significant effect
D Potential decreased exposure of ARV drug
E Potential elevated exposure of ARV drug
Numbers refer to increased or decreased AUC as observed in drug-drug interaction studies
ATV/c:
ATV co-formulated with COBI (300/150 mg qd)
DRV/c:
DRV co-formulated with COBI (800/150 mg qd)
CAB/RPV:
CAB and RPV im long acting injections
Es (estrogens)
COC (combined oral contraceptive)
EC (emergency contraception)
IP (implant)
IUD (intrauterine device)
POI (progestin only injectable)
POP (progestin only pill)
TS (transdermal patch)
VR (vaginal ring)
Interactions with ABC, FTC, 3TC, ZDV
ABC, FTC, 3TC, ZDV:
no clinically relevant interactions expected
Interactions with cabotegravir (oral)
None
Interactions with ibalizumab
None
Comments
- Alternative or additional contraceptive measures are recommended or, if used for hormone replacement therapy, monitor for signs of oestrogen deficiency.
- Unboosted ATV increased ethinylestradiol AUC by 48%. Use no more than 30 μg of ethinylestradiol if co-administered with unboosted ATV and at least 35 μg of ethinylestradiol if co-administered with ATV/r.
- Depending on the contraceptive method, ethinylestradiol concentrations are either not significantly changed (COC) or significantly decreased (VR). Levels of co-administered progestin are markedly decreased. Use with EFV is not recommended as it may impair contraceptive efficacy.
- Daily dose of ethinylestradiol should not exceed 30 μg. Caution is advised, particularly in persons with additional risk factors for thromboembolic events.
- European SmPC states a hormonal contraceptive should contain at least 30 μg ethinylestradiol.
- When used in a combination pill, the estrogen component is reduced to a small extent.
- When used in a combination pill, the estrogen component is significantly reduced, caution is recommended and additional contraceptive measures should be used.
- EFV is expected to decrease the progestin exposure and thereby impair the efficacy of the contraceptive method. A reliable method of barrier contraception must be used in addition to hormonal contraceptives.
- Clinical monitoring is recommended due to the potential for hyperkalaemia.
- Used in combination with ethinylestradiol (0.015 mg/day) which is predicted to be decreased. Since there is no possibility to adjust ethinylestradiol, caution is recommended and additional contraceptive measures should be used.
- A modeling study predicted a higher risk of having subtherapeutic medroxyprogesterone concentrations (i.e. <0.1 ng/mL) at week 12 in women with higher BMI on EFV treatment and even higher risk when EFV was given together with rifampicin. The risk of subtherapeutic concentrations is prevented by dosing medroxyprogesterone every 8-10 weeks in women with a higher body weight on EFV and particularly on efavirenz plus rifampicin.
- Concurrent use of TDF and medroxyprogesterone has been shown to increase bone mineral density loss compared to TDF alone.
- Unboosted ATV increased ethinylestradiol AUC by 48% and norethisterone AUC by 110%. Use no more than 30 μg of ethinylestradiol if co-administered with unboosted ATV and at least 35 μg of ethinylestradiol if co-administered with ATV/r.
- Unlikely to have clinical consequences as hormone is administered as single dose.
- Use 3 mg as a single dose for emergency contraception. Note, doubling the standard dose may be outside the product license in some regions, but a pharmacokinetic study showing that a 3 mg single dose of levonorgestrel compensated for the reduction in levonorgestrel supports this recommendation.
- Not recommended; non-hormonal emergency contraception (Cu-IUD) should be considered.
Further Information
For additional drug-drug interactions and for more detailed pharmacokinetic interaction data and dosage adjustments, please refer to: http://www.hiv-druginteractions.org (University of Liverpool)
