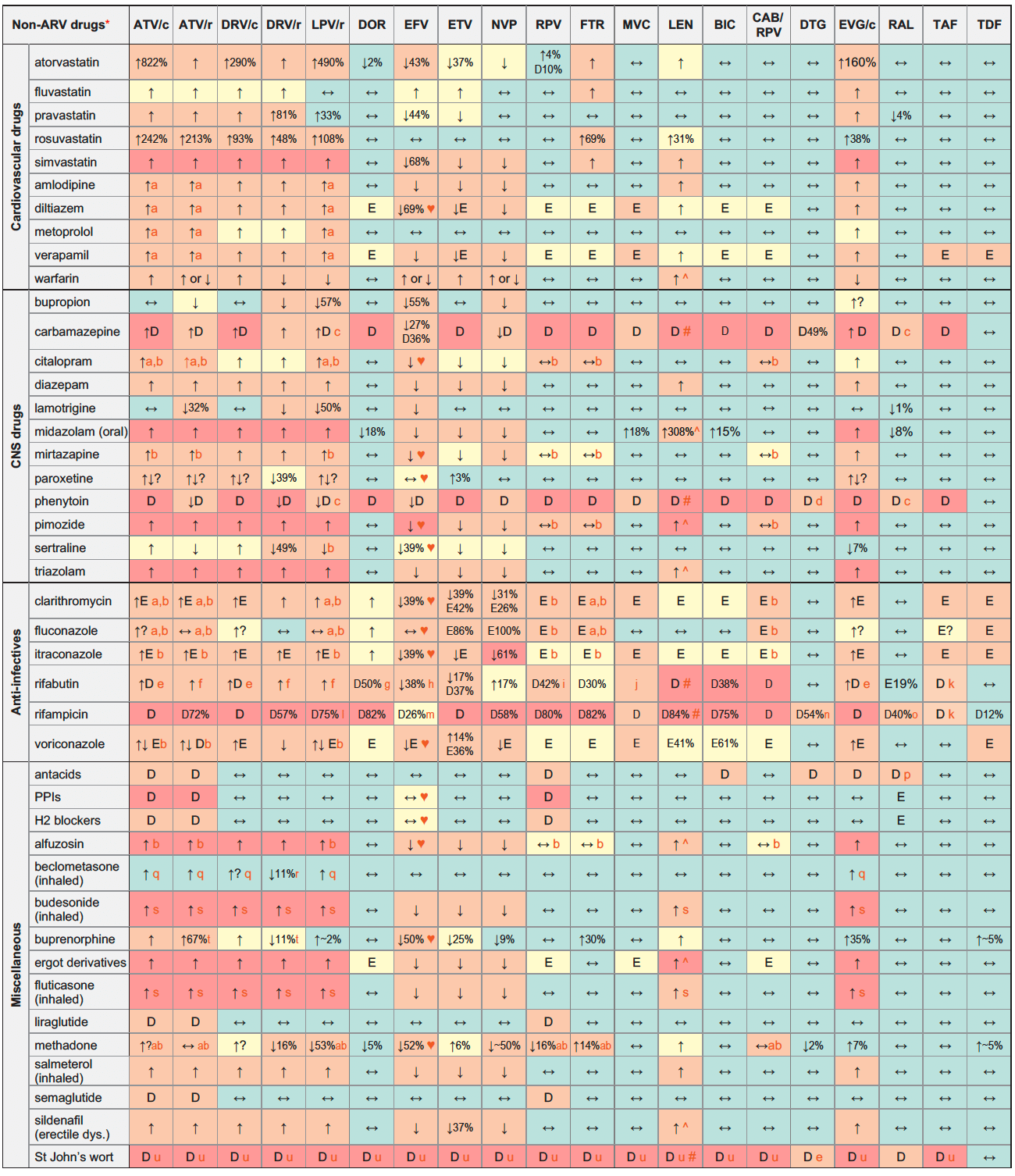
ARVs & non-ARVs

Legend
↑ Potential elevated exposure of the non-ARV drug
↓ Potential decreased exposure of the non-ARV drug
↔ No significant effect
D Potential decreased exposure of ARV drug
E Potential elevated exposure of ARV drug
Numbers refer to increased or decreased AUC as observed in drug-drug interaction studies
ATV/c:
Co-formulated with COBI (300/150 mg qd)
DRV/c:
Co-formulated with COBI (800/150 mg qd)
CAB/RPV:
CAB and RPV im long acting injections
* Table summarises the drug-drug interactions between HIV therapy and some commonly prescribed co-medicines as well as the drug-drug interactions of particular clinical relevance. This table is not exhaustive.
Interactions with ABC, FTC, 3TC, ZDV
ABC:
- Decreased ABC exposure with phenytoin, rifampicin;
- Decreased methadone exposure;
- Increased carbamazepine exposure
FTC, 3TC:
No clinically relevant interactions expected
ZDV:
- Decreased ZDV exposure with clarithromycin, rifampicin;
- Increased ZDV exposure with fluconazole, methadone;
- Increased carbamazepine exposure;
- Decreased phenytoin exposure
Interactions with cabotegravir (oral)
Decreased CAB exposure with carbamazepine, phenytoin, rifampicin (59%); these drugs should not be co-administered. Decreased CAB exposure with antacids; potentially clinically significant interaction.
Interactions with ibalizumab
None
Comments
- ECG monitoring is recommended.
- Caution as both drugs can induce QT interval prolongation.
- Co-administration with LPV/r 800/100 qd or RAL 1200 mg qd is not recommended. If use is unavoidable, give LPV/r 400/100 mg bid or RAL 400 mg bid, with monitoring of response.
- The European SmPC recommends DTG 50 mg bid in persons without INSTI resistance. The US Prescribing Information recommends that co-administration should be avoided as there are insufficient data to make dosing recommendations.
- Reduce rifabutin to 150 mg 3 times per week.
- Reduce rifabutin to 150 mg qd. Monitoring for rifabutin-related toxicities (i.e. uveitis or neutropenia) is advised with daily administration of rifabutin.
- The product label for DOR recommends to increase DOR dosage to 100 mg bid when co-administered with rifabutin. DOR should be kept at 100 mg bid for at least another 2 weeks following cessation of rifabutin due to the persisting inducing effect upon discontinuation of a moderate/ strong inducer.
- Increase rifabutin to 450 mg daily.
- The RPV dose should be increased to 50 mg qd during co-administration (and decreased to 25 mg qd when rifabutin is stopped). Note, it is recommended to maintain RPV 50 mg qd for at least another 2 weeks following cessation of rifabutin due to the persisting inducing effect upon discontinuation of a moderate/strong inducer.
- Increase MVC to 600 mg bid in the absence of a PI. Give MVC 150 mg bid with a PI.
- Rifamycins decrease the exposure of TAF when given 25 mg qd therefore the label recommends to use TAF 25 mg bid. However, the intracellular tenofovir diphosphate (active entity) concentrations are likely to be higher than those observed with TDF even without rifampicin suggesting that usage of TAF 25 mg qd with rifampicin, rifapentine or rifabutin may be acceptable.
- If no other option use RTV 400 mg bid or double dose LPV/r.
- EFV should be used at 600 mg qd in presence of rifampicin (in absence of rifampicin, EFV can be used at 400 mg qd or 600 mg qd).
- Administer DTG 50 mg bid in treatment-naïve or INSTI-naïve persons. This dose adjustment should be maintained for 2 weeks after stopping rifampicin as the inducing effect persists after discontinuation of a strong inducer. Alternative to rifampicin should be used where possible for INSTIexperienced persons with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance.
- RAL 400 or 800 mg bid.
- Al, Mg containing antacids not recommended with RAL 400 mg bid or 1200 mg qd. If co-administration with an antacid is unavoidable, calcium carbonate antacids can be used but only with RAL 400 mg bid.
- Increase in concentration of active metabolite observed with RTV 100 mg bid alone but without significant effect on adrenal function. Caution is still warranted, use the lowest possible corticosteroid dose and monitor for corticosteroid side effects.
- DRV/r decreased the exposure of active metabolite (beclometasone-17- monopropionate), no significant effect on adrenal function was seen.
- Risk of having elevated corticosteroid levels, Cushing's syndrome and adrenal suppression. This risk is present for oral and injected corticosteroid but also for topical, inhaled or eye drops administration.
- Concentrations of norbuprenorphine increased.
- A study suggests a low risk of a clinically relevant pharmacokinetic interaction with low-hyperforin formulations (< 1 mg/day) of St John’s Wort (hyperforin is the constituent responsible for induction of CYPs and P-gp). Co-administration may be considered with St John’s Wort formulations that clearly state the hyperforin content and which have a total daily hyperforin dose of 1 mg or less.
♥ EFV prolonged the QT interval above the regulatory threshold of concern in homozygous carriers of the CYP2B6*6/*6 allele (516T variant). Coadministration with a drug with a known risk of TdP is contraindicated in the EFV European label.
^ LEN causes moderate inhibition of CYP3A4 and, when discontinued, remains in the circulation for prolonged periods. Residual concentrations of LEN may affect the exposure of sensitive CYP3A4 substrates and/or narrow therapeutic index drugs that are initiated within 9 months after the last subcutaneous dose of LEN.
# At least a 2-week (moderate inducers) or 4-week (strong inducers) cessation period is recommended prior to initiation of LEN due to the persisting inducing effect after discontinuation of an inducer.
Further Information
For additional drug-drug interactions and for more detailed pharmacokinetic interaction data and dosage adjustments, please refer to: http://www.hiv-druginteractions.org (University of Liverpool)