Antihypertensives & ARVs
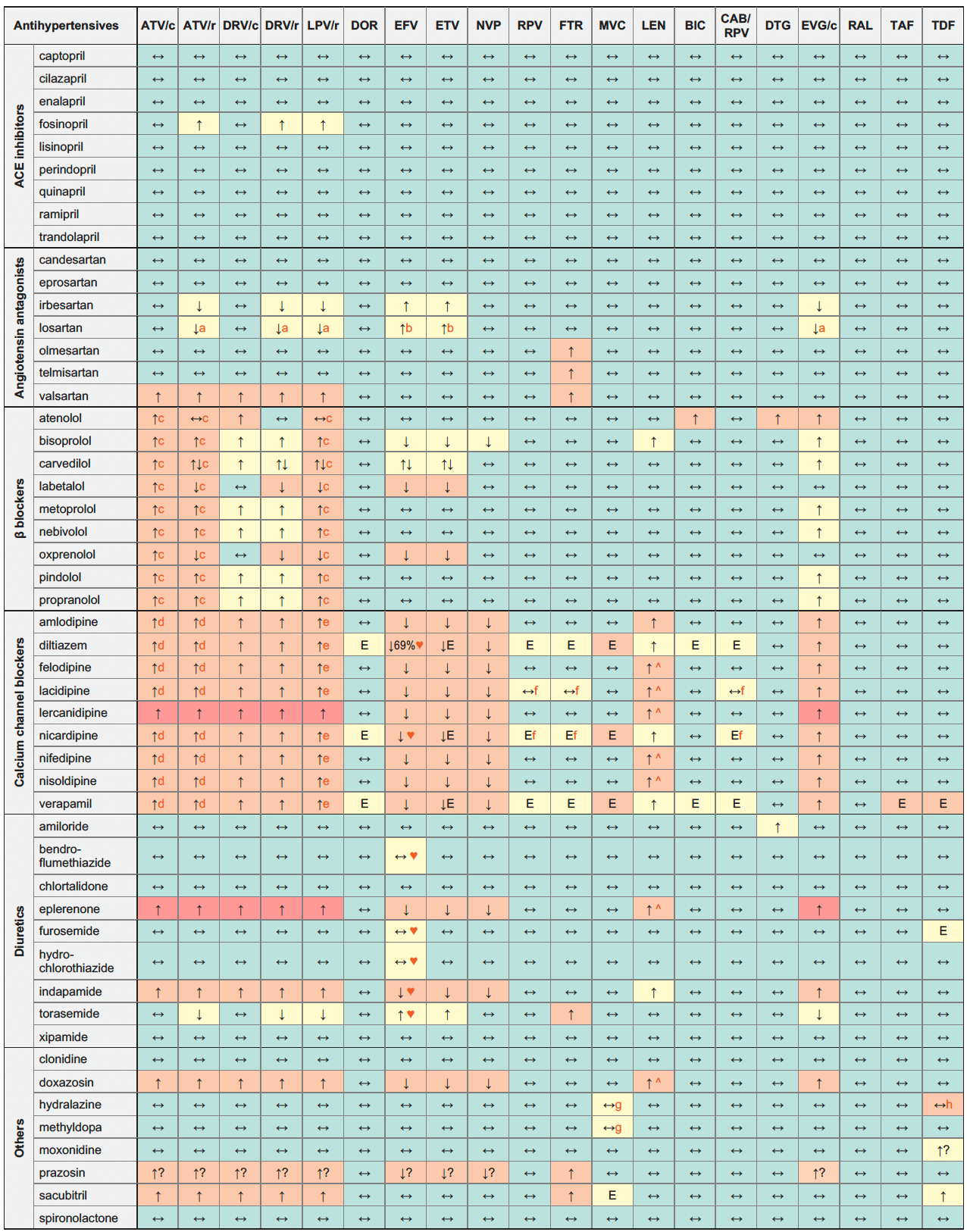
Legend
↑ Potential elevated exposure of the antihypertensive
↓ Potential decreased exposure of the antihypertensive
↔ No significant effect
D Potential decreased exposure of ARV drug
E Potential elevated exposure of ARV drug
Numbers refer to increased or decreased AUC as observed in drug-drug interaction studies
ATV/c:
ATV co-formulated with COBI (300/150 mg qd)
DRV/c:
DRV co-formulated with COBI (800/150 mg qd)
CAB/RPV:
CAB and RPV im long acting injections
Note: although some drug interactions are predicted to potentially require a dosage adjustment based on the drug's metabolic pathway, clinical experience with a particular antihypertensive and ARV drug may indicate that dosage adjustments are not an a priori requirement
Interactions with ABC, FTC, 3TC, ZDV
ABC, FTC, ZDV:
No clinically relevant interactions expected
3TC:
- Increased 3TC exposure with atenolol and amiloride;
- Increased exposure of atenolol and amiloride
Interactions with cabotegravir (oral)
None
Interactions with ibalizumab
None
Comments
- Parent drug concentrations decreased but active metabolite increased.
- Parent drug concentrations increased but active metabolite decreased.
- Risk of PR interval prolongation.
- ECG monitoring recommended.
- Use with caution as both LPV and calcium channel blockers prolong the PR interval. Clinical monitoring is recommended.
- Caution as both drugs can induce QT interval prolongation.
- Use with caution in persons with a history of postural hypotension or on concomitant medicinal products known to lower blood pressure, and those at increased risk of cardiovascular events.
- Hydralazine has some nephrotoxic potential. If co-administration is unavoidable, monitor renal function closely.
♥ EFV prolonged the QT interval above the regulatory threshold of concern in homozygous carriers of the CYP2B6*6/*6 allele (516T variant). Coadministration with a drug with a known risk of TdP is contraindicated in the EFV European label.
^ LEN causes moderate inhibition of CYP3A4 and, when discontinued, remains in the circulation for prolonged periods. Residual concentrations of LEN may affect the exposure of sensitive CYP3A4 substrates and/or narrow therapeutic index drugs that are initiated within 9 months after the last subcutaneous dose of LEN.
Further Information
For additional drug-drug interactions and for more detailed pharmacokinetic interaction data and dosage adjustments, please refer to: http://www.hiv-druginteractions.org (University of Liverpool)
